Vladimir Putin says Russia has approved ‘world first’ virus vaccine. But questions over its safety remain
Putin #Putin

“A vaccine against coronavirus has been registered for the first time in the world this morning,” Putin said on state TV. “I know that it works quite effectively, it forms a stable immunity.”
Putin added that one of his daughters had already taken it; he said she had a slightly higher temperature after each dose, but that: “Now she feels well.”
Developed by the Moscow-based Gamaleya Institute, the vaccine has been named Sputnik-V, a reference to the surprise 1957 launch of the world’s first satellite by the Soviet Union. It has yet to go through crucial Phase 3 trials where it would be administered to thousands of people.
The claim of victory by Putin in the global push to make an effective vaccine against Covid-19 comes amid suggestions that Russia has cut essential corners in its development.
Critics say the country’s push for a vaccine is partly due to political pressure from the Kremlin, which is keen to portray Russia as a global scientific force.


Russian President Vladimir Putin announced the approval of the Sputnik-V vaccine during a teleconference meeting with members of his government on August 11, 2020.
Russia has released no scientific data on its testing and CNN is unable to verify the vaccine’s claimed safety or effectiveness.
Despite this, Russian officials have told CNN that at least 20 countries and some US companies have expressed interest in the vaccine.
Kirill Dmitriev, head of the Russian Direct Investment Fund (RDIF), which is funding the vaccine research, said interest from other countries for over a billion doses of the vaccine had been received.
“We’ve seen considerable interest in the Russian vaccine developed by the Gamaleya Institute abroad. Moreover, we have received preliminary applications for over 1 billion doses of the vaccine from 20 countries,” he said Monday.
“Along with our foreign partners, we are already prepared to manufacture over 500 million doses of vaccine per year in five countries, and the plan is to ramp up production capacity even higher. So far, countries in Latin America, the Middle East and Asia have displayed the greatest interest in the vaccine, and we are about to finalize a number of contracts for the purchase of the vaccine.”
Dmitriev said Phase 3 trials of the vaccine would start Wednesday in Russia, and that they would also take place in other countries.
“We have already reached agreements on conducting the relevant trials of the Gamaleya vaccine [abroad] with partners from the UAE, Saudi Arabia, and a number of other countries,” he said.
Safety concerns

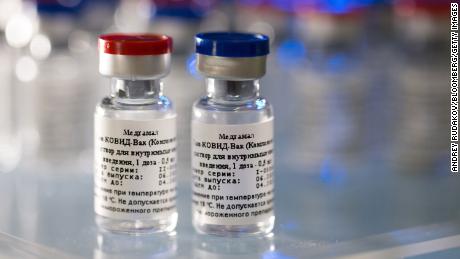
Vials containing the two components of Russia’s Covid-19 vaccine — named Sputnik-V — which has been developed by the Gamaleya Institute in Moscow.
Experts have voiced unease over Moscow’s rapid approval process for the vaccine.
“It is unclear precisely what is actually happening with the Russian vaccine,” Michael Head, Senior Research Fellow in Global Health at the University of Southampton in the UK, told the Science Medical Centre.
“It is vital that any vaccine roll-out has the confidence of the general public, and that there is good communication of the level of effectiveness and any likely side effects. At this point in time, there is no data on the Russian-led vaccine for the global health community to scrutinize.”
Danny Altmann, Professor of Immunology at Imperial College London, told the Science Media Centre there were concerns about releasing a vaccine before it was fully tested.
“The bar is necessarily set very high for criteria that must be satisfied for approval after Phase 3 clinical trials,” Altmann said. “The collateral damage from release of any vaccine that was less than safe and effective would exacerbate our current problems insurmountably. I hope these criteria have been followed. We are all in this together.”
But Moscow has brushed off concerns about the vaccine’s safety.
“According to the results, the vaccine has shown high efficiency and safety. All volunteers developed high [tiers] of antibodies to Covid-19, while none of them showed serious complications of immunization,” Russian Health Minister Mikhail Murashko said.
Other trials underway
Russia is just one of many countries rushing to produce a vaccine for Covid-19, which has now infected more than 20 million people, killing more than 730,000 around the world. 

An employee from Russia’s biotech company BIOCAD, which is developing its own coronavirus vaccine, and working on another one in cooperation with the country’s virus research centre.
There are 25 other vaccines in the clinical evaluation stage of development and a further 139 candidate vaccines in the preclinical evaluation stage according to the World Health Organization.
Closely watched vaccines in development include one from the University of Oxford and AstraZeneca and another from the biotechnology company Moderna and the US National Institute of Health. Both have showed promising results and are currently undergoing Phase 3 testing.
In June, the Chinese government approved the use of an experimental coronavirus vaccine for the country’s military. The vaccine, known as Ad5-nCoV, was jointly-developed by the Beijing Institute of Biotechnology — part of the Chinese government’s Academy of Military Medical Sciences — and vaccine company CanSino Biologics.
Previous results of Ad5-nCoV trials, published in medical journal the Lancet, were met with a lukewarm response by experts.
Earlier this month, the Kremlin denied allegations Russian spies hacked into American, Canadian and British research labs to steal vaccine development secrets.
This story has been updated.
CNN’s Matthew Chance and Ben Westcott contributed to this report.