Henrietta Lacks And Immortal Cell Lines
First Immortal #FirstImmortal

In early 1951, a woman named Henrietta Lacks visited the “colored ward” at Johns Hopkins hospital for a painful lump she found on her cervix. She was seen by Dr. Howard W. Jones, who indeed found a tumor growing on the surface of her cervix. He took a tissue sample, which confirmed Henrietta’s worst fears: She had cancer.
The treatment at the time was to irradiate the tumor with radium tubes placed in and around the cervix. The hope was that this would kill the cancerous cells while preserving the healthy tissue. Unbeknownst to Henrietta, a biopsy was taken during her radium procedure. Slivers of her tumor and of healthy cervix cells were cut away. The cancer cells were used as part of a research project. Then something amazing happened: the cancerous cells grew and continued to grow outside of her body.
As Henrietta herself lay dying, the HeLa immortal cell line was born. This cell line has been used in nearly every aspect of medical research since the polio vaccine. Millions owe their lives to it. Yet, Henrietta and her family never gave consent for any of this. Her family was not informed or compensated. In fact, until recently, they didn’t fully grasp exactly how Henrietta’s cells were being used.
The Life of Henrietta Lacks
 Loretta Pleasant was born on August 1, 1920, in Roanoke, Virginia. At some point, her name changed from Loretta to Henrietta, but the how and why is lost to family lore. After her mother died, she and her siblings were split up among other family members. Henrietta went to live with her grandfather and her cousin David in Clover, Virginia. Henrietta grew up working the same tobacco farms her ancestors had worked as slaves. She and David had their first child in 1935 and married a few years later. The couple moved to Turner Station, Maryland so David could work for the booming Bethlehem Steel company in Sparrows Crossing.
Loretta Pleasant was born on August 1, 1920, in Roanoke, Virginia. At some point, her name changed from Loretta to Henrietta, but the how and why is lost to family lore. After her mother died, she and her siblings were split up among other family members. Henrietta went to live with her grandfather and her cousin David in Clover, Virginia. Henrietta grew up working the same tobacco farms her ancestors had worked as slaves. She and David had their first child in 1935 and married a few years later. The couple moved to Turner Station, Maryland so David could work for the booming Bethlehem Steel company in Sparrows Crossing.
Henrietta had five children in total: Lawrence, Elsie, Deborah, David Jr, and Joseph. The family was poor, and for African Americans living in the Jim Crow era, life was hard. But Henrietta made sure they never wanted for anything. Sunday dinner was a special meal in the Lacks home. Henrietta always cooked a large meal and made bread pudding with the leftover bread she had baked that week. Cousins and newcomers to the neighborhood always had a place to stay in the Lacks home while they got on their feet.
 Henrietta and David Lacks
Henrietta and David Lacks
Before the birth of her youngest son Joseph, Henrietta told a cousin that she had a knot in her womb. That knot would eventually be diagnosed as the cancer which would kill her. She didn’t die peacefully. Her cancer was incredibly aggressive, and cancer treatments at the time were nowhere near what we are accustomed to today. By August 1951 the tumors had spread throughout her abdomen. The continued radiation treatments burned her inside and out, while she suffered indescribable pain.
By August 1951 she was admitted to the hospital and on October 4th, she passed away. David gave permission for an autopsy to be performed, and tumors which were white in color were found throughout her abdomen. They covered her internal organs like strings of pearls. Her bladder and kidneys had been almost completely replaced with cancerous tissue. Henrietta was laid to rest in Clover, in an unmarked grave near her mother. She was only 31 years old.
George Gey’s Pursuit of Immortal Cell Lines
 George Otto Gey was a cell biologist working at Johns Hopkins Medical Center. Gey could be thought of as an early modern biohacker. His lab was stocked with equipment he built himself from junkyard parts. Even the sinks were Gey’s own creation, handmade from stones he obtained from a local quarry. His lab was managed by his wife, Margaret — originally a surgical nurse. While George was a kid at heart, Margaret kept the lab running smoothly.
George Otto Gey was a cell biologist working at Johns Hopkins Medical Center. Gey could be thought of as an early modern biohacker. His lab was stocked with equipment he built himself from junkyard parts. Even the sinks were Gey’s own creation, handmade from stones he obtained from a local quarry. His lab was managed by his wife, Margaret — originally a surgical nurse. While George was a kid at heart, Margaret kept the lab running smoothly.
The two had spent years searching for a human immortal cell line — cells which could be reproduced infinitely. If a cell line like this could be found it would become a standard in medical research. Immortal lines had been created from animal cells, but never human.
The operating theory at the time was that cells in culture should be able to live — and multiply forever. In practice though, cell and tissue samples would always die. Teams around the world were attempting to figure out how to culture cells and keep them alive. Gey worked with witches brews of culture medium including chicken plasma, human umbilical cord blood, and other ingredients. George also built numerous tools to support cell growth, including the roller drum system. George would take any cells he could get his hands on, especially cancer cells. It’s not surprising then that a sample of Henrietta’s cells was sent to his lab.
Henrietta’s cancer cells did something no other human cells had ever done: They grew, and kept growing. Cell lines at the time were named with the first two letters of the patient’s first and last names. Henrietta Lacks was shortened to HeLa — the world’s first immortal human cell line.
 Lunchtime at the Gey Lab. Margaret Gey with Minnie, a technician.
Lunchtime at the Gey Lab. Margaret Gey with Minnie, a technician.
Gey had achieved his immediate goal of discovering a cell line, but it currently existed only in his lab. It couldn’t help researchers around the world if they didn’t have access to it. The problem was shipping cells. They had to travel via air freight, and even then they could die. Gey came up with a new medium and method of shipping frozen cells on ice. This allowed the cells to be shipped by regular mail. When received, the cells could be thawed and grown in an incubator.
The HeLa line was immediately put to use. Jonas Salk found that the cells were perfect for testing his polio vaccine. The first ever cell factory was created at Tuskegee University to keep Salk and other researchers supplied with cells. Eventually, commercial entities were formed to produce and sell the cell lines. Over subsequent years, millions of dollars were made by these companies while the Lacks family lived in poverty.
George Gey didn’t look for credit or payment for creating HeLa. While use of the line was becoming more widespread he was already off looking at new cell lines to culture. It took almost a year of pressure from Margaret and his team before he wrote a simple abstract about the discovery.
Gey himself died of Pancreatic cancer in 1970. Even as he was dying, he submitted himself for research studies, and tried to get his medical team to culture his own cancerous cells, hoping that his own cancer might form a new cell line.
Use and Misuse of the HeLa Immortal Cell Line 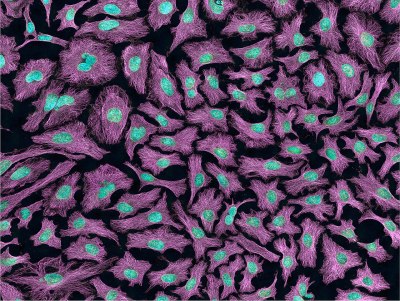 Multiphoton fluorescence image of HeLa cells with cytoskeletal microtubules (magenta) and DNA (cyan). From Wikipedia
Multiphoton fluorescence image of HeLa cells with cytoskeletal microtubules (magenta) and DNA (cyan). From Wikipedia
The HeLa cell line in cellular biology is as common a tool as a wrench is to a mechanic. In her 2009 book The Immortal Life of Henrietta Lacks, Rebecca Skloot found that over 60,000 scientific articles referenced HeLa, increasing at a rate of 300 each month. HeLa was used to create the polio vaccine, and in chemotherapy research. HeLa was key to mapping the human genome. In fact, it was a HeLa cell which was used to determine that humans have 46 chromosomes. HeLa cells went to space in both American and Russian satellites, and they accompanied Yuri Gagarin on his historic flight. The cells traveled to the moon with the Apollo missions, and were subjected to atomic bomb blasts to test the reaction of human cells.
There were some morally reprehensible uses of HeLa cells as well. Sloan Kettering immunologist Chester Southam wanted to see if the cells could infect other humans. He started with patients who already had cancer, injecting HeLa cells in their arms. The cells grew into tumors. The tumors were removed, but in several cases, they grew back. In one case Henrietta’s cancer metastasized to the patient’s lymph nodes. The same experiment was tried with volunteers from an Ohio prison. Once again, tumors grew in the prisoner’s arms. In this case, though, the prisoner’s healthy immune systems eventually fought off and rejected the HeLa cells.
By the end of his research, Southam had injected over 600 people with HeLa. Many of these people were gynecological surgery patients at hospitals where he worked. The patients had never given consent to be injected. For this, he was eventually brought up on charges of fraud, deceit, and unprofessional conduct. In 1963, The Regents of the University of the State of New York found him guilty, and he was placed on medical probation for one year.
Cross Contamination Risks During Cell Study 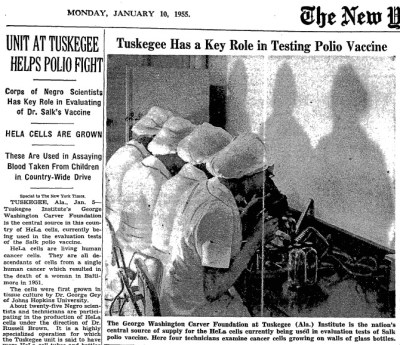 Tuskegee Cell Factory
Tuskegee Cell Factory
While the HeLa cell line has been incredibly beneficial for research, it needs to be handled carefully. HeLa multiplies so quickly that it will quickly destroy any other cell line it comes in contact with. Cross contamination has been and still is a huge problem. HeLa cells can travel on dust particles, through poorly cleaned lab tools, and on researchers’ gloves. Many millions of dollars of research have been lost due to the fact that the researchers were working on the wrong type of cells. The cells they intended to work on had been taken over by HeLa.
The contamination issue was first discovered by Stanley Gartler in 1967. Gartler found that many of the varied cell lines he was ordering from biological suppliers were testing positive for a specific genetic marker which is typically only found in HeLa. He presented his results and was widely criticized, as it would mean years of research was worthless. Scientists weren’t ready to throw away all that work.
In order to search for more genetic markers to identify HeLa cells, scientists went back to the source — Henrietta’s family. In the early 1970’s, the Lacks family was contacted and blood samples were taken. Henrietta’s children were never fully informed exactly what the blood was being used for. They thought they were being tested for the cancer which killed their mother.
Several more genetic markers for HeLa were derived from these tests. While it is now much easier to detect HeLa contamination, the contamination itself is still a problem. Even with modern filtration and sterilization techniques, HeLa cells can be found contaminating unrelated samples.
The Secret to HeLa’s Immortality
Henrietta’s cancer cells divide faster and were more hardy than any other human cells ever studied. What made them special though? First would be the cause of the cancer itself — Henrietta had HPV-18, a particularly nasty form of the Human Papillomavirus which is now known to cause cancer. She also had syphilis. This has been attributed to David Lacks’ infidelity.
Viruses inject parts of their genetic material into that of healthy cells. In Henrietta’s case, HPV-18 DNA was inserted into multiple sites of her cells’ DNA. HeLa cells can have anywhere from 76 to 80 chromosomes. Normal human cells have 23 pairs of chromosomes for a total of 46. These changes impacted many of the hard-coded genetic “programs” that the cells use to operate. For example, tumor suppression may have been turned off while cell growth rate was increased. Most importantly, one of these changes affected the mechanics of HeLa cell division.
The key to HeLa’s immortality is in the way cells divide. At the end of each chromosome is a repeating section of DNA called telomeres. As a normal cell divides, the number of telomeres gets smaller and smaller until it reaches a pre-set limit, called the Hayflick limit. At this point, a cell will no longer divide. When HeLa cells divide, they create an enzyme called telomerase. This enzyme prevents the telomeres from being reduced and allows the cell to divide an unlimited number of times. Telomeres are not completely understood — they also play a part in the aging of the overall organism. In humans, there are around 40,000 base pairs at birth, and only 11,000 base pairs in old age.
Moral Questions  The Lacks family with Rebecca Skloot
The Lacks family with Rebecca Skloot
While the HeLa cell line has been incredibly helpful for the human race, there are moral questions about how the cells were first obtained, and how they have been used. Henrietta Lacks never signed a form saying she allowed her cells to be used for research. Her family was never fully informed of the work done with Henrietta’s cells, or their own blood. Much of the Lacks’ family anger has been directed at Johns Hopkins and George Gey himself. However, neither Hopkins nor Gey ever made money from the cell line.
Through the years many companies have cultured and sold the HeLa cell line. None have compensated the Lacks family, who struggled to pay their own medical bills. The situation improved somewhat in 2009, with the publication of Rebecca Skloot’s book and the subsequent HBO movie. However, the Lacks still have to fight for their rights. In 2013, the entire genome of the HeLa line was sequenced and published without the family’s knowledge. The family did get involved and were able to come to an agreement with the research community to keep a single limited access database of the genome used for research only.
In America, laws have changed over the years and informed consent is now enforced. HIPAA laws protect a patient’s privacy. Yet the procedure performed on Henrietta would still be legal. When a piece of your body is cut away during a medical procedure, it is considered the property of the doctor and medical institution. This means medical professionals can do what they want with it, including selling it for profit. This was challenged and upheld in the 1990 Supreme Court case Moore v. Regents of the University of California. Genetic testing is a special case here, in the USA — the Genetic Information Nondiscrimination Act of 2008 protects against discrimination due to findings from genetic testing.
The Immortal Life of Henrietta Lacks  Deborah Lacks holding an image of HeLa cells
Deborah Lacks holding an image of HeLa cells
The story of Henrietta Lacks and the HeLa cell line went largely untold for decades. Even her name was spread as “Helen Lane” or “Helen Larson”. Henrietta’s own children and grandchildren had no idea the impact their mother’s cells were having on scientific and medical research. There were a few articles and one BBC TV special dedicated to the story, but it was largely unknown to the public. Over the years the family dealt with doctors asking for blood, science writers asking for a story, and even a con man who tried to use the situation for his own profit.
Rebecca Skloot, a science writer, became interested in the story. She worked closely with the Lacks family, especially Henrietta’s Daughter Deborah. While writing the book, Skloot promised to use proceeds from the book to form a foundation which would ensure the family had money for education. Her book The Immortal Life Of Henrietta Lacks was incredibly well researched and spent 75 weeks on the New York Times bestseller list. In 2017, it was released as an HBO movie, produced by and starring Oprah Winfrey as Deborah Lacks and Rose Byrne as Rebecca Skloot. Rebecca also made good on her promise. The Henrietta Lacks Foundation was formed in 2010 and has enabled Henrietta’s grandchildren to achieve their dreams.
References:
Skloot, Rebecca. The Immortal Life of Henrietta Lacks Crown/Archetype.
Lacks, Lawrence. HeLa Family Stories: Lawrence and Bobbette (A Short Memoir) HeLa Family Enterprise, LLC.