Elon Musk says Neuralink has implanted first brain chip in a human
Neuralink #Neuralink
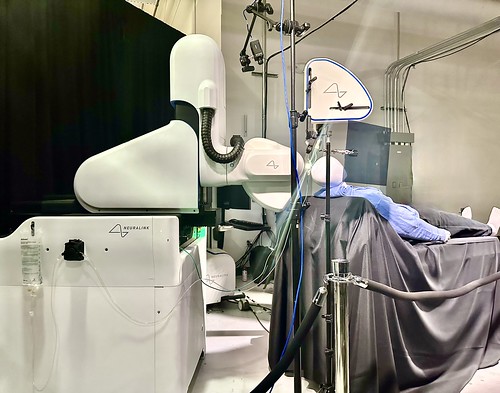
Elon Musk, Neuralink’s billionaire founder, said the first human received an implant from the brain-chip startup on Sunday and is recovering well, in a post on Twitter/X on Monday.
The US Food and Drug Administration (FDA) had given the company clearance last year to conduct its first trial to test its implant on humans.
“Initial results show promising neuron spike detection,” Musk added.
The startup’s Prime study is a trial for its wireless brain-computer interface to evaluate the safety of the implant and surgical robot.
The study will assess the functionality of the interface, which enables people with quadriplegia, or paralysis of all four limbs, to control devices with their thoughts, according to the company’s website.
Neuralink did not immediately respond to a request for further details.
Reuters reported earlier this month that Neuralink was fined for violating US Department of Transportation (DoT) rules regarding the movement of hazardous materials.
During inspections of the company’s facilities in Texas and California in February 2023, DoT investigators found the company had failed to register itself as a transporter of hazardous material, the agency’s records show.
They also found improper packaging of hazardous waste, including the flammable liquid Xylene. Xylene can cause headaches, dizziness, confusion, loss of muscle coordination and even death, according to the US Centers for Disease Control and Prevention.
Neuralink received FDA clearance last year for its first trial to test the company’s implant in humans, a critical milestone for the startup. Reuters reported in June that the company was valued as high as $5bn, based on private stock trades.
Neuralink announced the implant trial in September. The company said that during the study, a robot developed by the company will surgically place the implants’ “ultra-fine” threads that help transmit signals in participants’ brains.