Covid: Under-30s offered alternative to AstraZeneca jab
Under-30s #Under-30s

By Nick TriggleHealth correspondent
image copyrightGetty Images
Under-30s are to be offered an alternative Covid jab to the AstraZeneca vaccine due to the evidence linking it to rare blood clots, the UK’s vaccine advisory body says.
A review by drugs regulator MHRA found by the end of March 79 people in the UK had suffered rare blood clots after vaccination – 19 of whom had died.
The regulator said this was not proof the jab had caused the clots.
But it said the link was getting firmer.
Nearly two-thirds of the cases of rare clots were seen in women. The people who died were aged between 18 and 79.
The cases have been seen among 20 million doses of the AstraZeneca vaccine given in the UK.
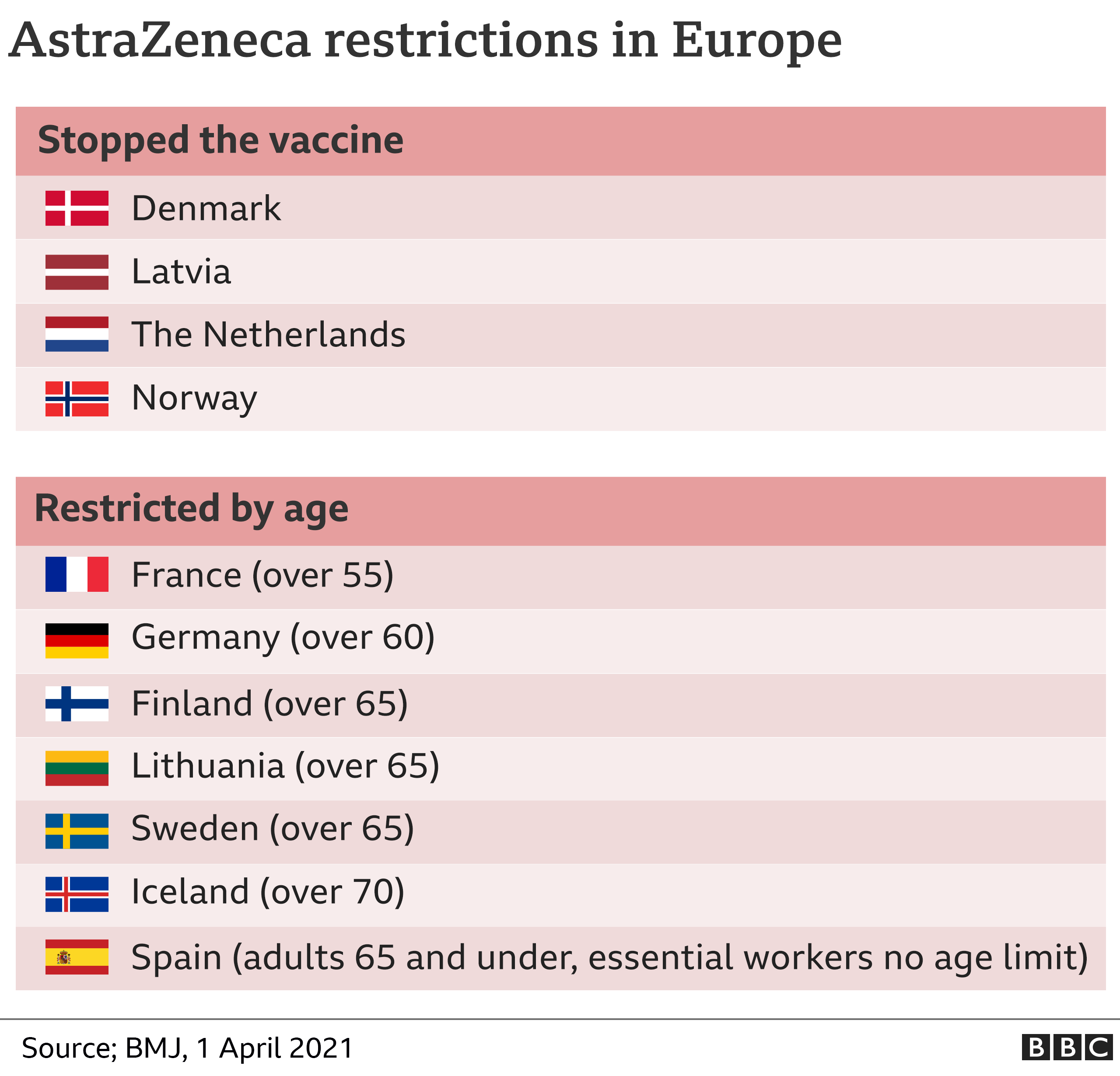
It comes as the EU’s medicines regulator says unusual blood clots should be listed as a very rare side effect of the jab, but that the benefits outweighed the risks. Some European countries have restricted the vaccine’s use.
Dr June Raine, chief executive of the MHRA, said the side-effects were “extremely rare” – and more work was going to identify if the vaccine was definitely causing the clots.
“The balance of benefits and known risks is still very favourable for the majority of people,” she said.
But she said for younger age groups it was more “finely balanced”.
She added: “The public’s safety is at the forefront of our minds.”
The review prompted the UK government’s vaccine advisory group, the JCVI, to recommend that people aged 18 to 29 be offered an alternative vaccine where available.
Prof Lim Wei Shen, of the JCVI, said the move was being made “out of the utmost caution” rather than any serious concerns.
![]()
People who have had their first dose of the AstraZeneca vaccine should still get their second dose, the MHRA said. Only those who suffered one of these rare blood clots after the first dose should not get vaccinated, it added.
People with blood disorders that leave them at risk of clotting should discuss the benefits and risks of vaccination with their doctor before going for a jab.
Anyone who suffers symptoms such as a persistent headache, blurred vision or confusion for four days or more after vaccination or who experience unusual skin bruising, shortness of breath or chest pain are being asked to seek medical advice.
England deputy chief medical officer Prof Jonathan Van-Tam described the move as a “course correction” – and said it was normal in medicine to change preferences in this way.
He also said the impact on the government’s promise to offer all adults a jab by the end of July should be “zero or negligible” as long as the expected supplies of the Pfizer and Moderna vaccines – the other two Covid vaccines in use in the UK – arrived as expected in the coming months.



